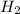Chemistry, 24.03.2020 16:19 juanaaraujo1104
Base your answer on the information below and your knowledge of chemistry. Methanol can be manufactured by a reaction that is reversible. In the reaction, carbon monoxide gas and hydrogen gas react using a catalyst. The equation below represents this system at equilibrium. CO(g) 2H2(g) CH3OH(g) energy. Explain, in terms of collision theory, why increasing the concentration of H2(g) in this system will increase the concentration of CH3OH(g).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Base your answer on the information below and your knowledge of chemistry. Methanol can be manufactu...
Questions

Mathematics, 21.10.2020 21:01

English, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01



History, 21.10.2020 21:01


Social Studies, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01




Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

History, 21.10.2020 21:01

Engineering, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01


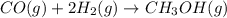
![Rate=k[CO]^x[H_2]^y](/tpl/images/0560/8875/426a0.png)
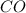 and
and