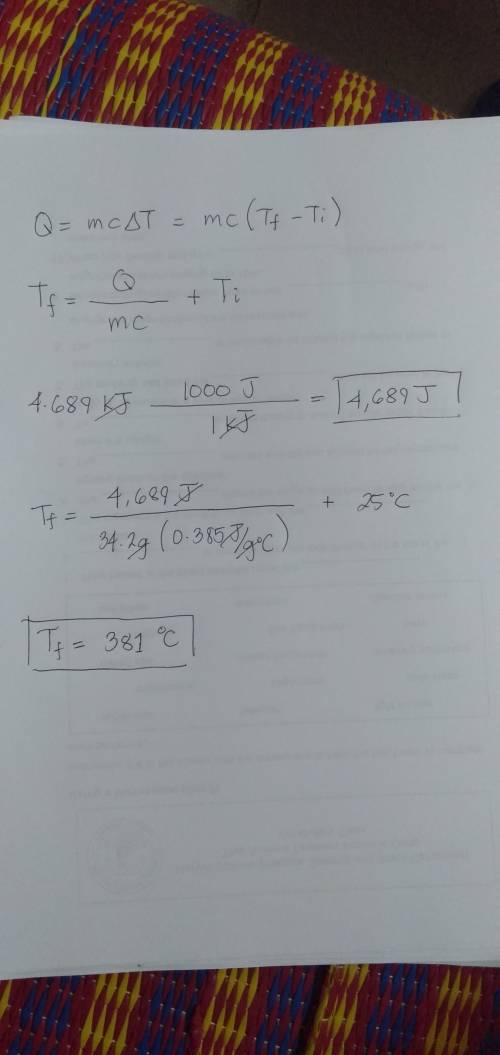Chemistry, 24.03.2020 14:03 paatnguyyen
The specific heat of copper is 0.385 J/(g °C). If 34.2 g of copper, initially at 25°C, absorbs 4.689 kJ, what will be the
final temperature of the copper?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
The specific heat of copper is 0.385 J/(g °C). If 34.2 g of copper, initially at 25°C, absorbs 4.689...
Questions




Health, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01


World Languages, 20.09.2020 03:01

Social Studies, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01




Mathematics, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01