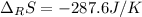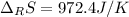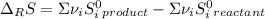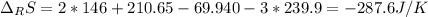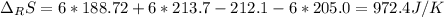Chemistry, 24.03.2020 05:55 giuliabril
Calculate ΔS°rxn (J/k) for 3NO2(g) + H2O(l)LaTeX: \longrightarrow⟶ NO(g) +2HNO3(l) C6H12O6(s) + 6O2(g) LaTeX: \longrightarrow⟶ 6H2O(g) +6CO2(g) Enter numbers to 1 decimal places. Substance or Ion S° (J/molLaTeX: \cdot⋅K) N2(g) 191.5 N2O(g) 219.7 NO(g) 210.65 NO2(g) 239.9 F2(g) 202.7 H2(g) 130.6 HNO3(l) 155.6 HNO3(aq) 146 H2O(l) 69.940 H2O(g) 188.72 C6H12O6(s) 212.1 O2(g) 205.0 CO2(g) 213.7 CO2(aq) 121 NF3(g) 260.6

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3


Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Calculate ΔS°rxn (J/k) for 3NO2(g) + H2O(l)LaTeX: \longrightarrow⟶ NO(g) +2HNO3(l) C6H12O6(s) + 6O2(...
Questions

Mathematics, 25.03.2021 01:00





English, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00

English, 25.03.2021 01:00

Arts, 25.03.2021 01:00


Chemistry, 25.03.2021 01:00




Mathematics, 25.03.2021 01:00


Arts, 25.03.2021 01:00