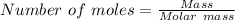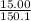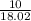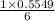Chemistry, 24.03.2020 05:29 lberman2005p77lfi
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limiting reactant is used up. Calculate the mass of H2S (34.08 g/mol) that can be produced from these reactants. Notice that you will need to balance the reaction equation.
___Al2S3(s)+ ___H2O > ___Al(OH)3(s)+ ___H2S(g)
a. 13.89 g
b. 10.21 g
c. 19.67 gd. 9.456 g
e. 1.108 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3


Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limitin...
Questions

Mathematics, 25.11.2020 01:50

Chemistry, 25.11.2020 01:50

Mathematics, 25.11.2020 01:50

History, 25.11.2020 01:50


Computers and Technology, 25.11.2020 01:50

Mathematics, 25.11.2020 01:50

Arts, 25.11.2020 01:50


Mathematics, 25.11.2020 01:50


Mathematics, 25.11.2020 01:50

English, 25.11.2020 02:00

Mathematics, 25.11.2020 02:00

Mathematics, 25.11.2020 02:00


English, 25.11.2020 02:00

Mathematics, 25.11.2020 02:00

Mathematics, 25.11.2020 02:00

Spanish, 25.11.2020 02:00