
Chemistry, 24.03.2020 05:24 Alizerodriguez2010
In a laboratory activity, a student titrates a 20.0 milliliter sample of Hcl(aq) using 0.025 M NAOH (aq). In one of the titration trails, 17.6 milliliters of the base solution exactly neutralizes the acid sample. Calculate the concentration f the hydrochloric acid using the titration data

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1


Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1
You know the right answer?
In a laboratory activity, a student titrates a 20.0 milliliter sample of Hcl(aq) using 0.025 M NAOH...
Questions





Mathematics, 11.12.2019 18:31






Mathematics, 11.12.2019 18:31


Social Studies, 11.12.2019 18:31

Geography, 11.12.2019 18:31




Mathematics, 11.12.2019 18:31


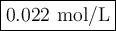

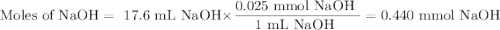
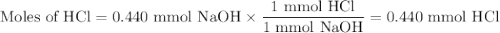
![\text{[HCl]} = \dfrac{\text{0.440 mmol HCl}}{\text{20.0 mL HCl}} = \textbf{0.022 mol/L}\\\text{The concentration of HCl is $\large \boxed{\textbf{0.022 mol/L}}$}](/tpl/images/0560/5762/e6247.png) }
}


