Chemistry, 24.03.2020 03:47 HTKPenguin
Calculate the pH of a buffer solution made by adding 15.0 g anhydrous sodium acetate (NaC2H3O2) to 100.0 mL of 0.200 M acetic acid. Assume there is no change in volume on adding the salt to the acid. (pKa for acetic acid is 4.74 or Ka is 1.8 x 10-5)3.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1



You know the right answer?
Calculate the pH of a buffer solution made by adding 15.0 g anhydrous sodium acetate (NaC2H3O2) to 1...
Questions

Mathematics, 22.04.2021 21:40

Mathematics, 22.04.2021 21:40

Mathematics, 22.04.2021 21:40


Mathematics, 22.04.2021 21:40


Mathematics, 22.04.2021 21:40


History, 22.04.2021 21:40

English, 22.04.2021 21:40

Mathematics, 22.04.2021 21:40

Chemistry, 22.04.2021 21:40







History, 22.04.2021 21:40

Mathematics, 22.04.2021 21:40

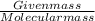
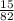
![\frac{[Salt]}{[Acid]}](/tpl/images/0560/4655/6743a.png)