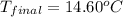Chemistry, 24.03.2020 03:28 bradleylogan78
A student carried heated a 25.00 g piece of aluminum to a temperature of 100°C, and placed it in 100.00 g of water, initially at a temperature of 10.0°C. Determine the final temperature of the system (aluminum and water)
cH2OB4.18J/gc
c Aluminum .900 j/g c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1


Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
A student carried heated a 25.00 g piece of aluminum to a temperature of 100°C, and placed it in 100...
Questions


Mathematics, 26.03.2021 17:40

Mathematics, 26.03.2021 17:40

Chemistry, 26.03.2021 17:40




Mathematics, 26.03.2021 17:40



Mathematics, 26.03.2021 17:40

English, 26.03.2021 17:40




Social Studies, 26.03.2021 17:40

English, 26.03.2021 17:40



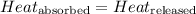
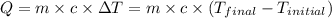
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0560/4279/09236.png) ......(1)
......(1)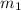 = mass of aluminium = 25.00 g
= mass of aluminium = 25.00 g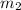 = mass of water = 100 g
= mass of water = 100 g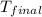 = final temperature = ?°C
= final temperature = ?°C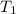 = initial temperature of aluminium = 100°C
= initial temperature of aluminium = 100°C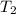 = initial temperature of water = 10°C
= initial temperature of water = 10°C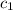 = specific heat of aluminium = 0.900 J/g°C
= specific heat of aluminium = 0.900 J/g°C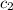 = specific heat of water= 4.18 J/g°C
= specific heat of water= 4.18 J/g°C![25\times 0.900\times (T_{final}-100)=-[100\times 4.18\times (T_{final}-10)]](/tpl/images/0560/4279/4bd6d.png)