

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

You know the right answer?
The equilibrium constant is equal to 5.00 at 1300 K for the reaction:2 SO2(g) + O2(g) ⇌ 2 SO3(g). If...
Questions





Mathematics, 28.03.2021 18:40

Mathematics, 28.03.2021 18:40

Mathematics, 28.03.2021 18:40


Mathematics, 28.03.2021 18:40

Social Studies, 28.03.2021 18:40

Mathematics, 28.03.2021 18:40


Mathematics, 28.03.2021 18:40

History, 28.03.2021 18:40

Mathematics, 28.03.2021 18:40


Mathematics, 28.03.2021 18:40


Mathematics, 28.03.2021 18:40

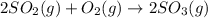
![Q=\frac{[SO_3]^2}{[SO_2]^2[O_2]}](/tpl/images/0560/3663/65406.png)
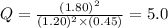
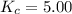
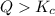 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.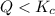 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.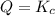 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

