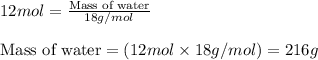Chemistry, 24.03.2020 02:44 musicaljay1276
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g) Which statement is correct about the complete combustion of 3.00 mole of propane, C3H8? \rm C_3H_8(g) + 5 O_2(g) --> 3 CO_2(g) + 4 H_2O(g)Which statement is correct about the complete combustion of 3.00 mole of propane, \rm C_3H_8?1. 12.00 mol H2O are produced.2. 3.00 g CO2 are produced.3. 3.00 mol CO2 are produced.4. 12.00 g H2O are produced

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g...
Questions

History, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31

Social Studies, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31


Mathematics, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31

Chemistry, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31



Mathematics, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31

Chemistry, 12.01.2020 08:31




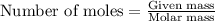 ......(1)
......(1)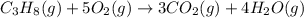
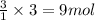 of carbon dioxide
of carbon dioxide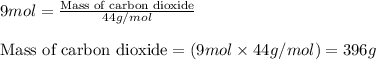
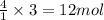 of water
of water