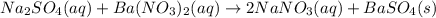Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2


Chemistry, 23.06.2019 19:00
Question 1 how do an ionic bond and a covalent bond differ? an ionic bond is an attraction between oppositely charged ions. a covalent bond is a sharing of electrons between atoms. a covalent bond is an attraction between oppositely charged ions. an ionic bond is a sharing of electrons between atoms. there is no difference. both an ionic bond and a covalent bond share electrons. there is no difference. both an ionic bond and a covalent bond are attractions between oppositely charged ions. question 2 what is the definition of a covalent bond? a bond between two positive ions a bond between two negative ions a bond between a positive and a negative ion a bond between two atoms question 3 what is a bond called that shares electrons between two neutral atoms? covalent bond ionic bond metallic bond polar bond question 4 water (h2o) is composed of the same elements as carbon monoxide (co). how do their properties compare? they have different properties because the arrangement of atoms is different. they have the same properties because they have the same atoms. they have different properties because they have different atoms. they have the same properties because they have the same arrangement of atoms. question 5 what is the definition of a chemical bond? a mutual attraction between the nuclei and electrons in two different atoms a mutual attraction between the nuclei and electrons in a single atom a mutual repulsion between the nuclei and electrons in two different atoms a mutual repulsion between the nuclei and electrons in a single atom
Answers: 1
You know the right answer?
Complete and balance the molecular equation for the reaction of aqueous sodium sulfate, Na2SO4,Na2SO...
Questions









Mathematics, 06.10.2020 14:01

English, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Spanish, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01

English, 06.10.2020 14:01



Mathematics, 06.10.2020 14:01