Chemistry, 24.03.2020 02:32 rainboworld3994
Suppose of barium acetate is dissolved in of a aqueous solution of sodium chromate. Calculate the final molarity of acetate anion in the solution. You can assume the volume of the solution doesn't change when the barium acetate is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
Suppose of barium acetate is dissolved in of a aqueous solution of sodium chromate. Calculate the fi...
Questions

Biology, 31.03.2021 23:00



Chemistry, 31.03.2021 23:00



Mathematics, 31.03.2021 23:00





Mathematics, 31.03.2021 23:00







History, 31.03.2021 23:00

Mathematics, 31.03.2021 23:00

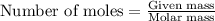
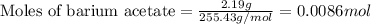
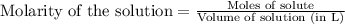 .....(1)
.....(1)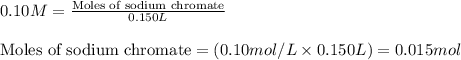
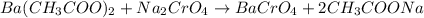
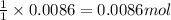 of sodium chromate
of sodium chromate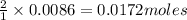 of sodium acetate
of sodium acetate