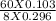Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

You know the right answer?
The p K a of hypochlorous acid is 7.530 . A 60.0 mL solution of 0.103 M sodium hypochlorite ( NaOCl...
Questions

Chemistry, 05.03.2022 21:30

Chemistry, 05.03.2022 21:30






Social Studies, 05.03.2022 21:30


English, 05.03.2022 21:40


Business, 05.03.2022 21:40


Mathematics, 05.03.2022 21:40

Mathematics, 05.03.2022 21:40



English, 05.03.2022 21:40


![\frac{[Conjugate base]}{[Acid]}](/tpl/images/0559/7901/225c1.png)