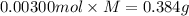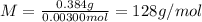A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrated with aqueous NaOH. If 30.0 mL of 0.100 M NaOH is required to reach the equivalence point, what is the molar mass of HA?
(a) 211 g/mol
(b) 128 g/mol
(c) 81.0 g/mol
(d) 37.0 g/mol
(e) 20.3 g/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrat...
Questions


Biology, 29.06.2019 11:30



Biology, 29.06.2019 11:30


History, 29.06.2019 11:30


Physics, 29.06.2019 11:30

Mathematics, 29.06.2019 11:30



Biology, 29.06.2019 11:30



Chemistry, 29.06.2019 11:30

History, 29.06.2019 11:30



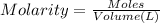
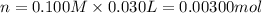
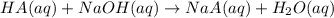
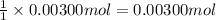 of HA
of HA