
Chemistry, 23.03.2020 21:45 angelina12386
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The entropy change when 1.85 moles of liquid Co vaporizes at 3097 °C, 1 atm is J/K.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2


You know the right answer?
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The...
Questions

Mathematics, 19.07.2020 18:01



Mathematics, 19.07.2020 18:01

Mathematics, 19.07.2020 18:01


Mathematics, 19.07.2020 18:01

Geography, 19.07.2020 18:01


Mathematics, 19.07.2020 18:01

Business, 19.07.2020 18:01

English, 19.07.2020 18:01


Mathematics, 19.07.2020 18:01



Mathematics, 19.07.2020 18:01


Chemistry, 19.07.2020 18:01

Mathematics, 19.07.2020 18:01

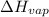 =
= 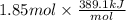
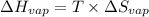
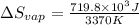
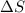 for vaporization is 213.6 J/K.
for vaporization is 213.6 J/K.


