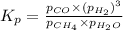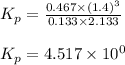Chemistry, 23.03.2020 21:09 darkskinnednune
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 1.5 L flask with 0.60 atm of methane gas and 2.6 atm of water vapor at 47. °C. He then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 1.4 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits x10.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions


Chemistry, 04.03.2021 19:50

History, 04.03.2021 19:50





English, 04.03.2021 19:50





Spanish, 04.03.2021 19:50

Mathematics, 04.03.2021 19:50


English, 04.03.2021 19:50

Mathematics, 04.03.2021 19:50




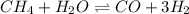
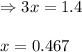
 for above equation follows:
for above equation follows: