
Chemistry, 23.03.2020 21:11 copelandgarret9972
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O] = 1.00 M comes to equilibrium at 25 °C ? Kc for the reaction is 0.0900.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 10:30
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
What concentration of Cl2O remains after a mixture that initially contains [H2O] = 1.00 M and [Cl2O]...
Questions


Geography, 21.07.2019 13:10




Mathematics, 21.07.2019 13:10


Biology, 21.07.2019 13:10


SAT, 21.07.2019 13:10

Computers and Technology, 21.07.2019 13:10

History, 21.07.2019 13:10




Health, 21.07.2019 13:10

Biology, 21.07.2019 13:10


Advanced Placement (AP), 21.07.2019 13:10

Physics, 21.07.2019 13:10

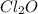 is 0.870 M.
is 0.870 M.![[H_2O]=1.00 M](/tpl/images/0559/4667/b0041.png)
![Cl_2O=[Cl_2O]=1.00 M](/tpl/images/0559/4667/4c14a.png)
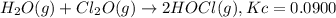
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0559/4667/da783.png)
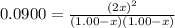
![[Cl_2O]=(1,00-x) M=1.00 M-0.130 M=0.870 M](/tpl/images/0559/4667/c9bd4.png)


