
Chemistry, 23.03.2020 20:35 mariahrpoulin9630
Sodium acetate spontaneously crystallizes out of a supersaturated solution upon standing or upon the addition of a seed crystal. Which of the following is true for the thermodynamic quantities of this system for thisprocess?(a)ΔS < 0J/K, ΔH < 0 J(b)ΔS < 0J/K, ΔH > 0 J(c)ΔS > 0J/K, ΔH < 0J(d)ΔS > 0J/K, ΔH > 0J

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1
You know the right answer?
Sodium acetate spontaneously crystallizes out of a supersaturated solution upon standing or upon the...
Questions



Social Studies, 25.09.2019 02:01

Biology, 25.09.2019 02:01




Physics, 25.09.2019 02:01

Social Studies, 25.09.2019 02:01


Mathematics, 25.09.2019 02:01


History, 25.09.2019 02:01



History, 25.09.2019 02:01


History, 25.09.2019 02:01

Biology, 25.09.2019 02:01

Social Studies, 25.09.2019 02:01

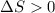 ).
).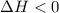 ).
).

