
Chemistry, 23.03.2020 20:21 ranaawilliamsoowl6dk
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemical equilibrium, which of the following explains what happens after the volume of the reaction mixture is increased (assume constant temperature)?
a. The amount of SO3(g) increases and the value for K increases.
b. The amount of SO3(g) decreases and the value for K increases.
c. The amount of SO3(g) stays the same and the value for K decreases.
d. The amount of SO3(g) decreases and the value for K stays the same.
e. The amount of SO3(g) increases and the value for K stays the same

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2


Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
You know the right answer?
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemica...
Questions



World Languages, 01.02.2020 00:46

Chemistry, 01.02.2020 00:46


Mathematics, 01.02.2020 00:46





History, 01.02.2020 00:47

History, 01.02.2020 00:47


Mathematics, 01.02.2020 00:47


History, 01.02.2020 00:47


Chemistry, 01.02.2020 00:47

Mathematics, 01.02.2020 00:47

Mathematics, 01.02.2020 00:47

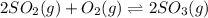
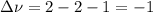
![K=\frac{[SO_3]_{eq}^2}{[SO_2]_{eq}^2[O_2]_{eq}}](/tpl/images/0559/3324/74387.png)


