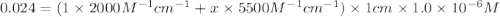The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5500 M-1cm-1. A protein has been isolated that is known to contain one tyrosine residue and an unknown number of tryptophans. A 1.0 micromolar solution of this protein is placed in a 1.0 cm cuvette and the absorbance at 280 nm is measured as 0.024. How many tryptophans are in the protein

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
You know the right answer?
The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5...
Questions


Mathematics, 21.02.2021 05:20

Mathematics, 21.02.2021 05:20

English, 21.02.2021 05:20

Arts, 21.02.2021 05:20

Business, 21.02.2021 05:20

Mathematics, 21.02.2021 05:20

Mathematics, 21.02.2021 05:20

Mathematics, 21.02.2021 05:20


Mathematics, 21.02.2021 05:20




Mathematics, 21.02.2021 05:20




Physics, 21.02.2021 05:20

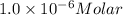


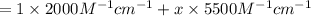
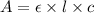 ( Beer-Lambert's law)
( Beer-Lambert's law)