
Cerium (IV) ions are strong oxidizing agents in acid ic solution, oxidizing arsenio us acid to arsen ic acid accor ding to the following equa tion:
2Ce4+(aq)+H3AsO3(aq)+3H2O(l)→2Ce3+( aq)+H3AsO4(aq)+2H+(aq)
A sample of As2O3 weighing 0.217 g is dissolved in basic solution and then acidified to make H3AsO3. Its titration with a solution of acidic cerium{IV) sulfate requires 21.47 ml. Determine the original concentration of Ce^4+(aq) in the titrating solution

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Cerium (IV) ions are strong oxidizing agents in acid ic solution, oxidizing arsenio us acid to arsen...
Questions




Mathematics, 16.12.2021 01:00


Geography, 16.12.2021 01:00



Mathematics, 16.12.2021 01:00




Mathematics, 16.12.2021 01:00

Social Studies, 16.12.2021 01:00


Chemistry, 16.12.2021 01:00


Arts, 16.12.2021 01:00


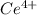 (aq) in the titrating solution.
(aq) in the titrating solution.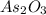 = 0.217 g
= 0.217 g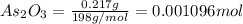
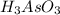 have 1 mole of As.
have 1 mole of As.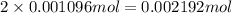 of
of 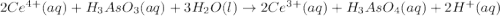
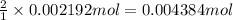 of cerium (IV) ions.
of cerium (IV) ions.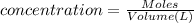
![[Ce^{4+}]=\frac{0.004384 mol}{0.02147 L}=0.2042 M](/tpl/images/0559/2089/fb855.png)



