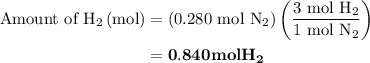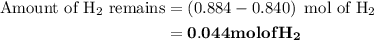Chemistry, 16.01.2020 10:31 balletbella0531
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia. n2(g)+3h2(g)=2nh3(g). assume 0.280 mol of n2 and 0.884 mol of h2 are present initially.. after complete reaction, how many moles of ammonia are . how many moles of h2 . how many moles of n2, what is the limiting or hydrogen)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia...
Questions


Mathematics, 06.11.2019 20:31





Mathematics, 06.11.2019 20:31

Geography, 06.11.2019 20:31

English, 06.11.2019 20:31

Health, 06.11.2019 20:31

Mathematics, 06.11.2019 20:31

Physics, 06.11.2019 20:31


Mathematics, 06.11.2019 20:31


Biology, 06.11.2019 20:31


Mathematics, 06.11.2019 20:31

Biology, 06.11.2019 20:31

Mathematics, 06.11.2019 20:31

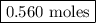
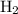 remain on completion of reaction is
remain on completion of reaction is 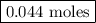
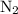 remain on completion of reaction is
remain on completion of reaction is 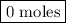
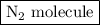
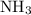 is as follows:
is as follows: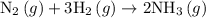
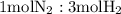
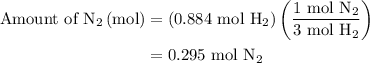
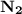 is present in limited quantity and is a limiting reagent.
is present in limited quantity and is a limiting reagent.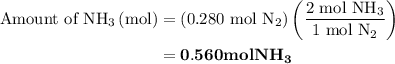
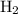 , therefore, the number of moles of
, therefore, the number of moles of