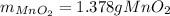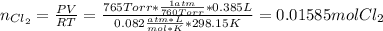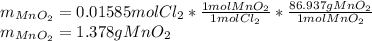Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq) , as described by the chemical equation
MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+ Cl2(g)
How much MnO2(s) should be added to excess HCl(aq) to obtain 385 mL Cl2(g) at 25 °C and 765 Torr ? mass of MnO 2

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3


Chemistry, 23.06.2019 12:40
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

Mathematics, 05.05.2020 16:23

Mathematics, 05.05.2020 16:23

Mathematics, 05.05.2020 16:23

History, 05.05.2020 16:23







English, 05.05.2020 16:23




Mathematics, 05.05.2020 16:23

Mathematics, 05.05.2020 16:23



Mathematics, 05.05.2020 16:23

Social Studies, 05.05.2020 16:23