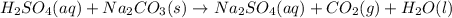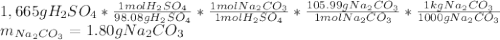Chemistry, 23.03.2020 16:51 tryintopassenioryear
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The emergency spill kit contained a full 2.0 kg bottle of sodium carbonate (105.99 g/mol). Is this enough sodium carbonate to neutralize the acid, according to the following reaction
H2SO4(aq) + Na2CO3(s) → Na2SO4(aq) + CO2(g) + H2O(l)
A. Yes, there is more than enough sodium carbonate.
B. Yes, there is exactly enough sodium carbonate-but no excess.
C. No, there is not enough sodium carbonate, but the amount is only about 10% too small.
D. No, there is not nearly enough sodium carbonate.
E. No, the reaction will start going backwards.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The e...
Questions

Social Studies, 20.07.2021 06:50




Mathematics, 20.07.2021 06:50






Mathematics, 20.07.2021 06:50


Social Studies, 20.07.2021 06:50

Mathematics, 20.07.2021 06:50

Social Studies, 20.07.2021 06:50

Mathematics, 20.07.2021 06:50



Chemistry, 20.07.2021 06:50

Chemistry, 20.07.2021 06:50