
Chemistry, 23.03.2020 03:32 amandajbrewerdavis
Zinc reacts with iodine in a synthesis reaction. Using a balanced chemical equation for the reaction, determine the percent yield of a 129.0gram sample of zinc was used and 510.6 grams of product is recovered

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

You know the right answer?
Zinc reacts with iodine in a synthesis reaction. Using a balanced chemical equation for the reaction...
Questions





Mathematics, 31.10.2019 18:31








Physics, 31.10.2019 18:31


Mathematics, 31.10.2019 18:31





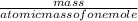
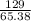
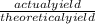 x100 equation 1
x100 equation 1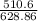 x 100
x 100

