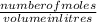Chemistry, 23.03.2020 03:00 williamabigan
Calculate the change in pH of a 1.00 L of a buffered solution preparing by mixing 0.50 M acetic acid (Ka = 1.8 x 10^-5) and 0.50 M sodium acetate when 0.010 mole of NaOH is added. Compare this pH change with that when the same amount 0.010 mole of NaOH was added to 1.00 L of water. (Remember what we did in class a week ago, the pH will jump from 7 to 12 when that tiny amount of NaOH was added).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Calculate the change in pH of a 1.00 L of a buffered solution preparing by mixing 0.50 M acetic acid...
Questions


Computers and Technology, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30


Mathematics, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

World Languages, 03.05.2021 18:30




History, 03.05.2021 18:30


Mathematics, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

Arts, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

Mathematics, 03.05.2021 18:30

![\frac{[base]}{[acid]}](/tpl/images/0558/5974/a552b.png)
![\frac{[0.5]}{[0.5]}](/tpl/images/0558/5974/ae4bf.png)