
Chemistry, 22.03.2020 22:50 jilliandantuma84
How many particles of Na are there in 1.43g of a molecular compound with a Molar mass of 23g?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
How many particles of Na are there in 1.43g of a molecular compound with a Molar mass of 23g?...
Questions

Mathematics, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01





Computers and Technology, 24.06.2020 23:01





English, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01

Biology, 24.06.2020 23:01


Business, 24.06.2020 23:01


History, 24.06.2020 23:01

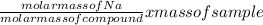
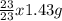
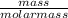 =
= 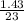 = 0.062mole
= 0.062mole 

