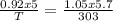A sample of argon has a volume of 5.0 dm^3 and the pressure is 0.92 atm.
If the final temperat...

Chemistry, 22.03.2020 02:39 dragon2998
A sample of argon has a volume of 5.0 dm^3 and the pressure is 0.92 atm.
If the final temperature is 30.° C, the final volume is 5.7 L, and the final
pressure is 800. mm Hg, what was the initial temperature of the argon?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
Questions

Mathematics, 14.02.2021 23:10



Mathematics, 14.02.2021 23:10

Mathematics, 14.02.2021 23:10

Physics, 14.02.2021 23:10

Computers and Technology, 14.02.2021 23:10



Mathematics, 14.02.2021 23:10



Biology, 14.02.2021 23:10

Mathematics, 14.02.2021 23:10

Mathematics, 14.02.2021 23:10


Mathematics, 14.02.2021 23:10

Mathematics, 14.02.2021 23:10

Biology, 14.02.2021 23:10

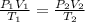
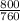 = 1.05atm
= 1.05atm