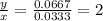Chemistry, 21.03.2020 10:33 odriskel49
The amounts of C and O are the same so the empirical formula has equal numbers of C and O atoms; that is, it has the general form CxHyOx. Determine the ratio of H/C (that is, y/x).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
The amounts of C and O are the same so the empirical formula has equal numbers of C and O atoms; tha...
Questions

Mathematics, 22.03.2021 07:20

Mathematics, 22.03.2021 07:20






English, 22.03.2021 07:20

Chemistry, 22.03.2021 07:20


Computers and Technology, 22.03.2021 07:20

English, 22.03.2021 07:20


Spanish, 22.03.2021 07:20


Mathematics, 22.03.2021 07:20

English, 22.03.2021 07:20


History, 22.03.2021 07:20

Computers and Technology, 22.03.2021 07:20

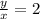
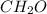
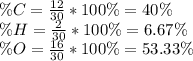
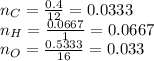
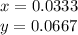
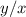 will be:
will be: