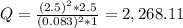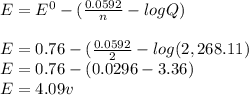Refer to the following standard reduction half-cell potentials at 25∘C:
VO2+(aq)+Ni2+(aq)2H+(aq)++2e−e−→ →Ni(s)VO2+(aq) +H2O(l)E∘=−0.23V E∘=0.99V
An electrochemical cell is based on these two half-reactions: Oxidation:Reduction:
Ni(s)VO2+(aq,0.083M)+2H+(aq,1.1M)+e −→→Ni2+(aq,2.5M)+2e−VO2+(aq,2.5M)+H 2O(l)
Calculate the cell potential under these nonstandard concentrations.
Express the cell potential to two decimal places and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
Refer to the following standard reduction half-cell potentials at 25∘C:
VO2+(aq)+Ni2+(a...
VO2+(aq)+Ni2+(a...
Questions



History, 14.02.2022 18:10


Mathematics, 14.02.2022 18:10



Mathematics, 14.02.2022 18:10

Social Studies, 14.02.2022 18:10


Mathematics, 14.02.2022 18:10

Mathematics, 14.02.2022 18:10





History, 14.02.2022 18:10


Mathematics, 14.02.2022 18:10

Advanced Placement (AP), 14.02.2022 18:10

![E = E^{0} - [(\frac{0.0592}{n} · log Q)]](/tpl/images/0557/5710/84c6b.png)
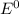 = Cell potential (standard conditions)
= Cell potential (standard conditions)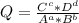
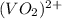 + e- -->
+ e- --> 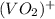 -0.23
-0.23 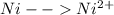 + 2 e- +0.99
+ 2 e- +0.99 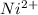 + 2e- 0.76 v
+ 2e- 0.76 v ![Q = \frac{[(VO_{2}+]^{2}* [Ni^{2+} }{[VO_{2+}]^{2} * Ni }]](/tpl/images/0557/5710/56e6b.png)
![[VO_{2} ^{2+}] = 2.5 M](/tpl/images/0557/5710/bdce1.png)
![[VO_{2}+] = 0.083 M](/tpl/images/0557/5710/9505a.png)
![[Ni^{2+}] = 2.5 M](/tpl/images/0557/5710/3a699.png)
![[Ni] = 1 M, it is a pure solid, so its activity in Q is unit (1). It is also applied for pure liquids.](/tpl/images/0557/5710/4494d.png)