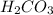Chemistry, 21.03.2020 08:24 BigGirlsTheBest
A mixture of a carboxylic acid and a phenol can often be separated by extracting with aqueous sodium bicarbonate and a suitable organic solvent. What difference in chemical properties of the carboxylic acid and the phenol make this separation possible?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

You know the right answer?
A mixture of a carboxylic acid and a phenol can often be separated by extracting with aqueous sodium...
Questions

Geography, 21.04.2021 14:00



Advanced Placement (AP), 21.04.2021 14:00

World Languages, 21.04.2021 14:00

English, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00



Chemistry, 21.04.2021 14:00

History, 21.04.2021 14:00

History, 21.04.2021 14:00

Physics, 21.04.2021 14:00



Geography, 21.04.2021 14:00

History, 21.04.2021 14:00


History, 21.04.2021 14:00

Chemistry, 21.04.2021 14:00

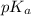 of carboxylic acid lies in the range of 1.92 - 4.76
of carboxylic acid lies in the range of 1.92 - 4.76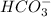 acts as a strong base because it is derived from weak conjugate acid
acts as a strong base because it is derived from weak conjugate acid