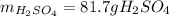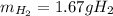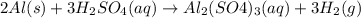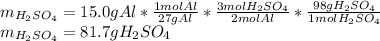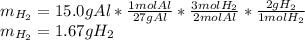Sulfuric acid dissolves aluminum metal according to the following reaction:
2Al (s) + 3H2SO4...

Chemistry, 21.03.2020 05:32 dalton200166
Sulfuric acid dissolves aluminum metal according to the following reaction:
2Al (s) + 3H2SO4 (aq) --> Al2(SO4)3 (aq) + 3H2 (g)
Suppose you wanted to dissolve an aluminum block with a mass of 15.0 g.
What minimum mass of H2SO4 (in g) would you need?
What mass of H2 gas (in g) would be produced by the complete reaction of the aluminum block?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1


Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
Questions


Computers and Technology, 08.09.2020 23:01


Mathematics, 08.09.2020 23:01


English, 08.09.2020 23:01



Mathematics, 08.09.2020 23:01


English, 08.09.2020 23:01





History, 08.09.2020 23:01