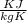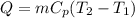Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
A sample of xenon gas at a pressure of 817 mm Hg and a temperature of 66 °C, occupies a volume of 13...
Questions

Mathematics, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40

English, 07.06.2021 19:40


Geography, 07.06.2021 19:40



Mathematics, 07.06.2021 19:40


Mathematics, 07.06.2021 19:40

Biology, 07.06.2021 19:40

History, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40


Mathematics, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40

Social Studies, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40

English, 07.06.2021 19:40

Chemistry, 07.06.2021 19:40

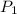 = 109 k pa = Final pressure
= 109 k pa = Final pressure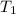 = 66°c = 339 K
= 66°c = 339 K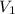 = 13.9 liter = 0.0139
= 13.9 liter = 0.0139 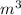
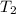 = 362 K
= 362 K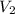 = 14.84 liter
= 14.84 liter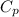 = 0.158
= 0.158