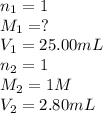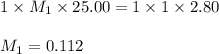Chemistry, 21.03.2020 03:13 ellisc7044
1. Determine the volume of 1M NaOH that is required to reach the equivalence point with 25.00 mL of HCl (of unknown concentration). From there, calculate the original concentration of the unknown HCl solution.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1
You know the right answer?
1. Determine the volume of 1M NaOH that is required to reach the equivalence point with 25.00 mL of...
Questions



Mathematics, 07.05.2021 05:00

Mathematics, 07.05.2021 05:00

Mathematics, 07.05.2021 05:00



Mathematics, 07.05.2021 05:00

English, 07.05.2021 05:00



Mathematics, 07.05.2021 05:00

Mathematics, 07.05.2021 05:00







Spanish, 07.05.2021 05:00


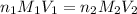
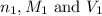 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 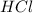
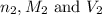 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.