
Chemistry, 21.03.2020 02:02 daartist3121
2 H2+O2=2 H2O
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H2O was produced?
Show your work.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

You know the right answer?
2 H2+O2=2 H2O
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H...
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H...
Questions



Mathematics, 09.01.2020 00:31



Mathematics, 09.01.2020 00:31


English, 09.01.2020 00:31




Computers and Technology, 09.01.2020 00:31



Advanced Placement (AP), 09.01.2020 00:31



History, 09.01.2020 00:31

Mathematics, 09.01.2020 00:31

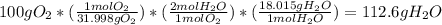
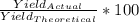 =
= 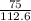 = 66.60403646
= 66.60403646


