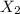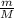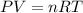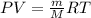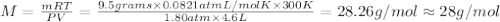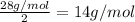To identify a diatomic gas (X2), a researcher carried out the following experiment: She weighed an empty 4.6-L bulb, then filled it with the gas at 1.80 atm and 27.0 ∘C and weighed it again. The difference in mass was 9.5 g . Identify the gas. Express your answer as a chemical formula. View Available Hint(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
To identify a diatomic gas (X2), a researcher carried out the following experiment: She weighed an e...
Questions

English, 05.06.2020 05:57

World Languages, 05.06.2020 05:57

English, 05.06.2020 05:57

Mathematics, 05.06.2020 05:57

Mathematics, 05.06.2020 05:57


Health, 05.06.2020 05:57



Mathematics, 05.06.2020 05:57


Mathematics, 05.06.2020 05:57

Geography, 05.06.2020 05:57

History, 05.06.2020 05:57

History, 05.06.2020 05:57


Mathematics, 05.06.2020 05:57


Mathematics, 05.06.2020 05:57

History, 05.06.2020 05:57

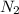 .
.