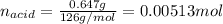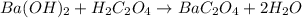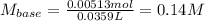Chemistry, 20.03.2020 11:54 jadenpmoore2008
Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary standard. Its formula is H2C2O4•2H2O. A student dissolves 0.647 grams of H2C2O4•2H2O in water and titrates the resulting solution with a solution of barium hydroxide of unknown concentration. If 35.9 mL of the barium hydroxide solution are required to neutralize the acid, what is the molarity of the barium hydroxide solution ?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1
You know the right answer?
Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary stan...
Questions

Arts, 19.08.2019 22:00


Physics, 19.08.2019 22:00


English, 19.08.2019 22:00

History, 19.08.2019 22:00

Mathematics, 19.08.2019 22:00




Biology, 19.08.2019 22:00

Mathematics, 19.08.2019 22:00

Mathematics, 19.08.2019 22:00





English, 19.08.2019 22:00

History, 19.08.2019 22:00

English, 19.08.2019 22:00