

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1


Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of...
Questions

History, 10.05.2021 20:50



Mathematics, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50


Mathematics, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50



Social Studies, 10.05.2021 20:50

Mathematics, 10.05.2021 20:50


History, 10.05.2021 20:50




Mathematics, 10.05.2021 20:50


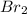 present in the reaction vessel.
present in the reaction vessel. = 0.682 mole
= 0.682 mole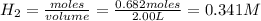
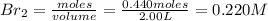
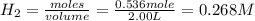
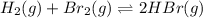
![K_c=\frac{[HBr]^2}{[Br_2]\times [H_2]}](/tpl/images/0556/0201/f7e28.png)
![[Br_2]=\frac{moles}{volume}\\0.147=\frac{xmole}{2.00L}\\\\x=0.294 mole](/tpl/images/0556/0201/4871d.png)


