
Chemistry, 20.03.2020 09:59 karmaxnagisa20
Determine the number of grams of hcl that can react with 0.750 g of Al(OH)3 according to the following reaction Al(OH)₃(s)+3HCl(aq)→AlCl₃(aq)+3H₂O( aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Determine the number of grams of hcl that can react with 0.750 g of Al(OH)3 according to the followi...
Questions

Mathematics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00


Mathematics, 01.10.2019 22:00


Biology, 01.10.2019 22:00

Social Studies, 01.10.2019 22:00

Biology, 01.10.2019 22:00


Computers and Technology, 01.10.2019 22:00

Physics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00

Biology, 01.10.2019 22:00

Physics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00

History, 01.10.2019 22:00


Social Studies, 01.10.2019 22:00


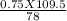 = 1.05 g of HCl.
= 1.05 g of HCl. 


