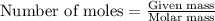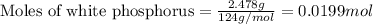Chemistry, 20.03.2020 10:03 xXCoryxKenshinXx
2.478 g of white phosphorus was used to make phosphine according to the equation: P₄(s) + 3OH⁻(aq) + 3H₂O(l) → PH₃(g) + 3H₂PO₂⁻(aq) Calculate the amount, in mol, of white phosphorus

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

You know the right answer?
2.478 g of white phosphorus was used to make phosphine according to the equation: P₄(s) + 3OH⁻(aq) +...
Questions




English, 08.12.2020 05:20


Mathematics, 08.12.2020 05:20

Mathematics, 08.12.2020 05:20

History, 08.12.2020 05:20

English, 08.12.2020 05:20

Mathematics, 08.12.2020 05:20

Social Studies, 08.12.2020 05:20



Mathematics, 08.12.2020 05:20

Mathematics, 08.12.2020 05:20



Mathematics, 08.12.2020 05:20

Social Studies, 08.12.2020 05:20

Chemistry, 08.12.2020 05:20