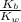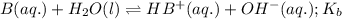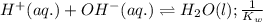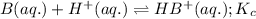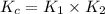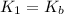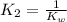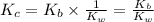Chemistry, 20.03.2020 10:23 briseno138
Given the following equilibrium constants: Kb B(aq) + H2O(l) ⇌ HB+(aq) + OH−(aq) 1/Kw H+(aq) + OH−(aq) ⇌ H2O(l) What is the equilibrium constant for the following reaction equal to? B(aq) + H+(aq) ⇌ HB+(aq)

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Given the following equilibrium constants: Kb B(aq) + H2O(l) ⇌ HB+(aq) + OH−(aq) 1/Kw H+(aq) + OH−(a...
Questions

Mathematics, 29.01.2021 06:20

Social Studies, 29.01.2021 06:20



Social Studies, 29.01.2021 06:20

Mathematics, 29.01.2021 06:20

Mathematics, 29.01.2021 06:20

Mathematics, 29.01.2021 06:20

History, 29.01.2021 06:20

Spanish, 29.01.2021 06:20

Advanced Placement (AP), 29.01.2021 06:20

Mathematics, 29.01.2021 06:20


Chemistry, 29.01.2021 06:20


Mathematics, 29.01.2021 06:20


English, 29.01.2021 06:20


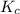 for the net reaction is
for the net reaction is