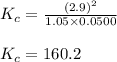Chemistry, 20.03.2020 09:07 khalilh1206
The following reaction was performed in a sealed vessel at 772 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.90M and [I2]=2.95M . The equilibrium concentration of I2 is 0.0500 M . What is the equilibrium constant, Kc, for the reaction at this temperature?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
The following reaction was performed in a sealed vessel at 772 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, on...
Questions


Advanced Placement (AP), 11.10.2020 09:01



Physics, 11.10.2020 09:01

History, 11.10.2020 09:01


Mathematics, 11.10.2020 09:01





Mathematics, 11.10.2020 09:01

Social Studies, 11.10.2020 09:01


Mathematics, 11.10.2020 09:01


History, 11.10.2020 09:01


Mathematics, 11.10.2020 09:01

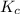 for the given equation is 160.2
for the given equation is 160.2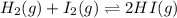
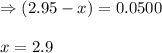
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0555/7227/62646.png)