
Chemistry, 20.03.2020 08:30 darwin59651
Calculate the pH of a 0.437 M aqueous solution of hydrocyanic acid (HCN, Ka = 4.0×10-10) and the equilibrium concentrations of the weak acid and its conjugate base.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 21.06.2019 13:30
Lithium diisopropylamide [(ch3)2ch]2nli, referred to as lda, enjoys many uses as a strong base in synthetic organic chemistry. it is customarily prepared by the reaction of diisopropylamine [(ch3)2ch]2nh with butyllithium. draw the products of the reactions in the appropriate boxes and select the acid, base, conjugate acid, and conjugate base. be sure to answer all parts.
Answers: 2

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

You know the right answer?
Calculate the pH of a 0.437 M aqueous solution of hydrocyanic acid (HCN, Ka = 4.0×10-10) and the equ...
Questions






Mathematics, 04.05.2021 07:30

English, 04.05.2021 07:30


Mathematics, 04.05.2021 07:30

History, 04.05.2021 07:30

History, 04.05.2021 07:30



Mathematics, 04.05.2021 07:30

Mathematics, 04.05.2021 07:30





![[HCN]_{eq}=0.43699M](/tpl/images/0555/6850/8a21f.png)
![[CN^-]_{eq}=1.322x10^{-5}M](/tpl/images/0555/6850/83c77.png)
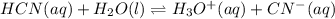
![Ka=\frac{[H^+]_{eq}[CN^-]_{eq}}{[HCN]_{eq}}](/tpl/images/0555/6850/4425b.png)
 due to the reaction extent, goes:
due to the reaction extent, goes: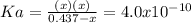
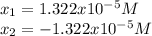
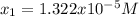
![pH=-log([H^+])=-log(1.322x10^{-5})=4.88](/tpl/images/0555/6850/f810b.png)
![[HCN]_{eq}=0.437M-1.322x10^{-5}M=0.43699M](/tpl/images/0555/6850/d4f3f.png)



