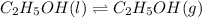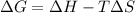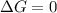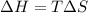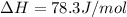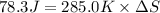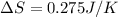Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Calculate the standard entropy of vaporization of ethanol, C2H5OH, at 285.0 K, given that the molar...
Questions




Mathematics, 23.06.2019 12:00

Biology, 23.06.2019 12:00

Mathematics, 23.06.2019 12:00

Mathematics, 23.06.2019 12:00

History, 23.06.2019 12:00

Biology, 23.06.2019 12:00

Mathematics, 23.06.2019 12:00

Biology, 23.06.2019 12:00

Mathematics, 23.06.2019 12:00

Biology, 23.06.2019 12:00

Mathematics, 23.06.2019 12:00




Chemistry, 23.06.2019 12:00


Mathematics, 23.06.2019 12:00