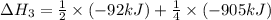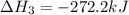Chemistry, 20.03.2020 07:19 valeriegarcia12
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia:
N2(g) + 3H2(g) →2NH3(g)
ΔH=−92.kJ
In the second step, ammonia and oxygen react to form nitric oxide and water:
4NH3(g) + 5O2(g) → 4NO(g) +6H2O(g)
ΔH=−905.kJ
Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step,...
Questions

Mathematics, 09.09.2021 04:30

Mathematics, 09.09.2021 04:30


History, 09.09.2021 04:30

Health, 09.09.2021 04:30

English, 09.09.2021 04:30


Health, 09.09.2021 04:30


English, 09.09.2021 04:30



English, 09.09.2021 04:30


Mathematics, 09.09.2021 04:30

History, 09.09.2021 04:30




History, 09.09.2021 04:30

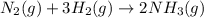 (1)
(1)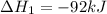
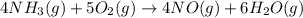 (2)
(2)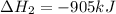
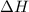 for the following reaction i.e,
for the following reaction i.e,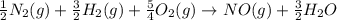
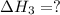
 for the reaction will be:
for the reaction will be: