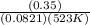A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 would be 0.50 atm at 523 K. However, PCl5 decomposes to gaseous PCl3 and Cl2, and the actual pressure in the flask was found to be 0.78 atm. Calculate Kp for the decomposition reaction PCl5(g) PCl3(g) Cl2(g) at 523 K. Also, calculate K at this temperature.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
A sample of gaseous PCl5 was introduced into an evacuated flask so that the pressure of pure PCl5 wo...
Questions




English, 19.06.2020 02:57


Mathematics, 19.06.2020 02:57




Computers and Technology, 19.06.2020 02:57


Mathematics, 19.06.2020 02:57








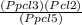 Kp =
Kp = 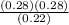 Kp = 0.35
Kp = 0.35