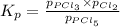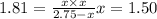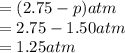At 250°C the equilibrium constant (Kp) for the reaction below is 1.81. PCl5(g) PCl3(g) + Cl2(g) Sufficient PCl5 is put into a vessel to give an initial pressure of 2.75 atm at 250°C. What will be the final pressure at this temperature after the system has reached equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3


Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
At 250°C the equilibrium constant (Kp) for the reaction below is 1.81. PCl5(g) PCl3(g) + Cl2(g) Suff...
Questions







Computers and Technology, 19.03.2020 21:45



English, 19.03.2020 21:45

History, 19.03.2020 21:45


English, 19.03.2020 21:45





English, 19.03.2020 21:46


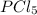 at equilibrium is 1.25 atm
at equilibrium is 1.25 atm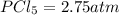
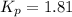
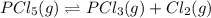
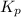 for the given reaction
for the given reaction