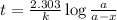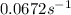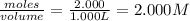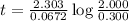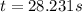At a certain fixed temperature, the reaction A(g) + 2 B(g) → AB2(g) is found to be first order in the concentration of A and zero order in the concentration of B. The reaction rate constant is 0.0672 s−1 . If 2.000 moles of A and 4.000 moles of B are placed in a 1.000 liter container, how many seconds will elapse before the concentration of A has fallen to 0.300 mol/liter? 1. 36.6948 2. 60.226 3. 63.2373 4. 34.244 5. 49.9242 6. 23.8631 7. 51.2735 8. 29.6425 9. 36.1356 10. 28.231 Answer in units of s.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
At a certain fixed temperature, the reaction A(g) + 2 B(g) → AB2(g) is found to be first order in th...
Questions



Health, 21.10.2019 17:40

English, 21.10.2019 17:40

English, 21.10.2019 17:40

Biology, 21.10.2019 17:40

History, 21.10.2019 17:40

Mathematics, 21.10.2019 17:40

Mathematics, 21.10.2019 17:40


Business, 21.10.2019 17:40

Mathematics, 21.10.2019 17:40

Chemistry, 21.10.2019 17:40

Mathematics, 21.10.2019 17:40

Biology, 21.10.2019 17:40




English, 21.10.2019 17:40

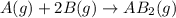
![Rate=k[A]^1[B]^0](/tpl/images/0555/2030/f8c47.png)