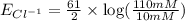Chemistry, 20.03.2020 01:57 Randomkid0973
1. Using the Nernst equation, calculate the equilibrium potential for Ca21 and for Cl2 from the following sets of data: a. Given [Ca21]o 5 1 mM, [Ca21]i 5 100 nM, find ECa21 b. Given [Cl2]o 5 110 mM, [Cl2]i 5 10 mM, find ECl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
1. Using the Nernst equation, calculate the equilibrium potential for Ca21 and for Cl2 from the foll...
Questions

Biology, 25.11.2019 05:31

Physics, 25.11.2019 05:31




Biology, 25.11.2019 05:31



History, 25.11.2019 05:31

Health, 25.11.2019 05:31


History, 25.11.2019 05:31


Chemistry, 25.11.2019 05:31


History, 25.11.2019 05:31




Chemistry, 25.11.2019 05:31

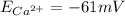 and
and 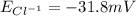
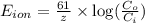
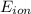 = equilibrium potential
= equilibrium potential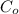 = concentration of the ion outside the cell
= concentration of the ion outside the cell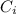 = concentration of the ion inside the cell
= concentration of the ion inside the cell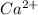 = 2
= 2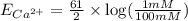
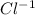 = 1
= 1