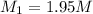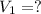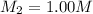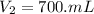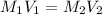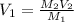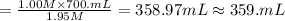A chemist must prepare 700.mL of 1.00M aqueous calcium bromide CaBr2 working solution. He'll do this by pouring out some 1.95M aqueous calcium bromide stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the calcium bromide stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
A chemist must prepare 700.mL of 1.00M aqueous calcium bromide CaBr2 working solution. He'll do this...
Questions




Mathematics, 30.04.2021 21:10


Computers and Technology, 30.04.2021 21:10


Mathematics, 30.04.2021 21:10


Mathematics, 30.04.2021 21:10

Mathematics, 30.04.2021 21:10


History, 30.04.2021 21:10

Computers and Technology, 30.04.2021 21:10

Mathematics, 30.04.2021 21:10


Spanish, 30.04.2021 21:10

Mathematics, 30.04.2021 21:10

History, 30.04.2021 21:10