Chemistry, 20.03.2020 01:29 latinotimo7643
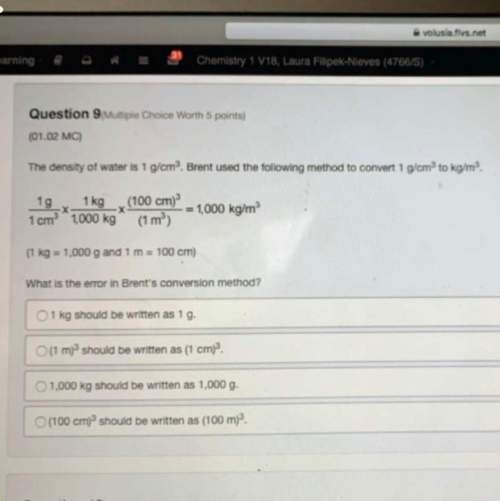
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g) 3CO2(g) + 4H2O (g)When 2.5 mol of O2are consumed in thisreaction, mol of CO2are produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g)...
Questions

Mathematics, 09.11.2020 23:00


History, 09.11.2020 23:00

English, 09.11.2020 23:00

English, 09.11.2020 23:00

Mathematics, 09.11.2020 23:00


English, 09.11.2020 23:00




History, 09.11.2020 23:00


Mathematics, 09.11.2020 23:00


Engineering, 09.11.2020 23:00




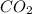 are produced.
are produced.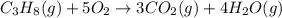
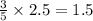 moles of carbon dioxide
moles of carbon dioxide